In the absence of positive biomarkers or following the development of drug resistance, immunotherapies, such as immune checkpoint inhibitors, have become an integral part of the treatment of advanced and metastatic NSCLC.
Despite these advances, NSCLC remains the leading cause of cancer-related deaths worldwide. In 2017 alone, in the US, NSCLC accounted for more cancer-related deaths than prostate, breast, brain, and colorectal cancers combined (Siegel et al. 2023). In response, the emergence of next-generation therapeutics, including bispecific antibodies (bsAbs), is providing significant promise for the future treatment of NSCLC.
In this blog, we discuss how bsAbs are gaining traction as a novel treatment strategy for NSCLC and showing great promise in combination therapy in clinical trials.
Non-Small Cell Lung Cancer — A Prominent Problem
NSCLC is caused by genetic mutations that lead to dysregulated control of cellular housekeeping mechanisms and uncontrolled cell growth. Mutations in the gene encoding the epidermal growth factor receptor (EGFR), which regulates cell proliferation, are among the most common in NSCLC, with one study reporting these mutations in 14% of NSCLC cases in Europe, particularly at exon 20 (Zhang et al. 2016). Additionally, mutations in the mesenchymal epithelial transition (MET) gene contribute to uncontrolled cell growth and resistance to targeted therapies with EGFR inhibitors in EGFR-positive patients.
With this increasingly comprehensive understanding of disease pathogenesis, what are the therapeutic options available in the clinic?
Challenges with Standard NSCLC Treatment Strategies
Surgery to remove cancerous cells is a common initial route during the treatment of early-stage NSCLC. For stage I NSCLC, adjuvant chemotherapy, immunotherapy, or targeted therapy might be used to target the remaining cancer cells as there is a higher risk of the cancer coming back, but this is not without unwanted side effects and may not induce the desired response. Radiotherapy may also be used post-surgery if appropriate. In later stage NSCLC, treatment can involve substantial removal of tissue, but a combination of neoadjuvant chemotherapy, with or without immunotherapy and radiotherapy is often administered. Immunotherapies, such as immune checkpoints inhibitors, have transformed the treatment of advanced and metastatic NSCLC in the last decade, demonstrating superior efficacy compared to chemotherapy alone in some patients (Desai and Peters 2023). However, outcomes remain suboptimal with many patients failing to respond or develop resistance over time.
Consequently, despite the advances in immunotherapy, the need for novel therapeutic approaches to treat NSCLC remains. bsAbs are gaining momentum in NSCLC, marking the next generation of cancer biotherapeutics with the potential to bring newfound capabilities to fight back against NSCLC.
Engineering Multimodal Bispecific Antibodies
Since their conception in the 1960s, clinical interest in bsAbs has grown rapidly due to their promising therapeutic potential and unique targeting capabilities (Riethmüller 2012). Although structurally similar to monovalent antibodies, bsAbs are engineered to have two distinct binding sites allowing the simultaneous recognition and binding of two antigens (Figure 1a, b).
bsAbs can work through multiple mechanisms, including immune cell engagement, tumor-associated antigen targeting, spatial drug payload delivery, or immune checkpoint blockade. While these approaches have been used with great success in the treatment of hematological cancers, their effectiveness in treating solid tumors (making up roughly 90% of all cancers), such as NSCLC, has been less impressive (Gu and Zhao 2024). Of the 14 globally approved bsAb therapies, only five are used against solid tumors. As such, their use in the clinic has been limited.
However, recent trials have aimed to address these limitations and develop effective bsAb-based therapies for solid tumor cancers.
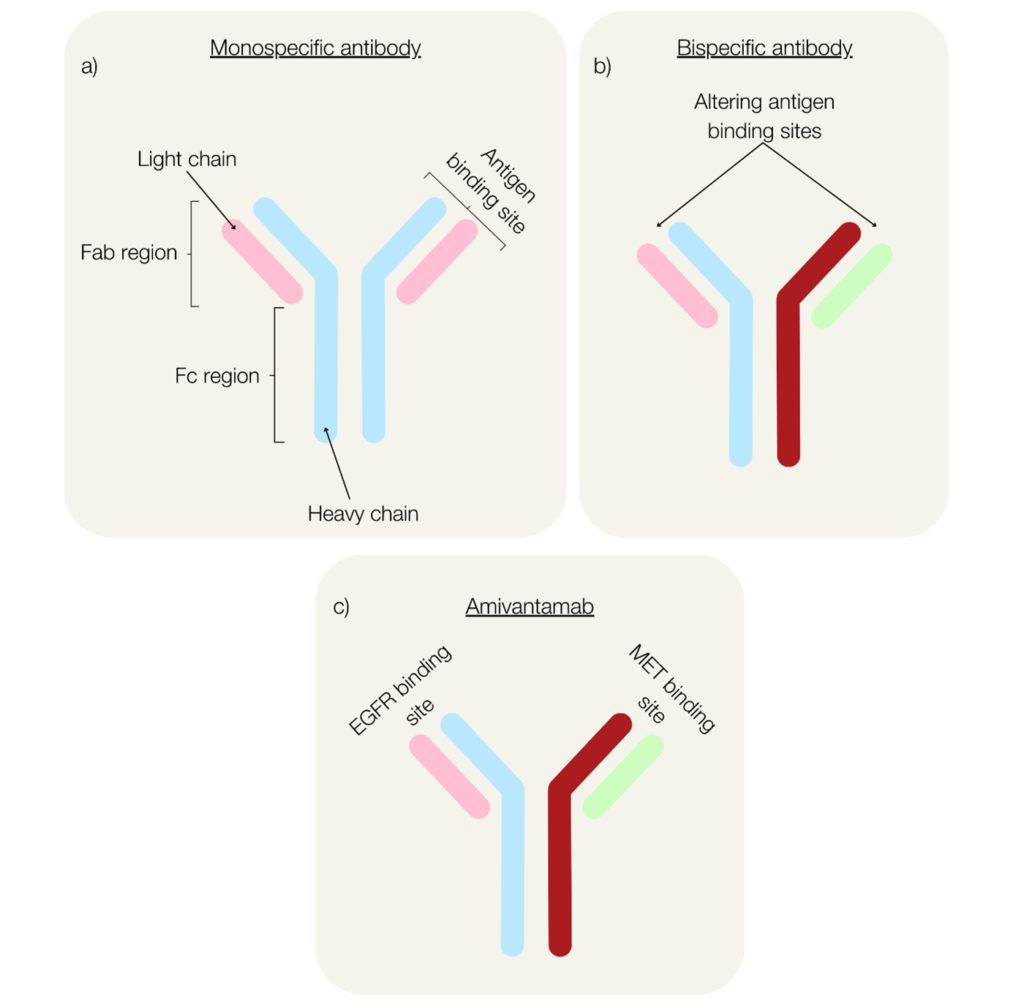 Fig. 1. Structure of monospecific antibodies (a), bispecific antibodies (b), and the NSCLC immunotherapy, amivantamab (c).
Fig. 1. Structure of monospecific antibodies (a), bispecific antibodies (b), and the NSCLC immunotherapy, amivantamab (c).
Amivantamab: A Potential Game-Changer for NSCLC Treatment
The FDA recently approved the use of amivantamab for patients with NSCLC whose disease had progressed on or after platinum-based chemotherapy following promising findings from the CHRYSALIS Phase I study (Park et al. 2021). Amivantamab, an immune-directing bsAb, works against EGFR exon 20 positive NSCLC by targeting EGFR and c-MET (Figure 1c) to block ligand-induced phosphorylation and reduce tumor size through signal inhibition (Gu and Zhao 2024).
The efficacy of amivantamab was later investigated in combination with chemotherapy, compared with chemotherapy treatment alone, in the PAPILLON clinical trial (Zhou 2023). Primary outcomes were defined as progression-free survival and key secondary outcomes included overall survival time, response duration, and symptomatic progression-free survival. The study revealed significantly reduced disease progression in the patients receiving amivantamab plus chemotherapy vs chemotherapy alone, with 33% vs 69% demonstrating cancer progression. Objective response in the combination therapy group was 73% vs 47% in the chemotherapy group and median progression-free survival was over 11 months vs only 6.7 months, respectively. Physical measurements of tumor pathology showed a mean reduction in size of 53% in the amivantamab plus chemotherapy group compared with 34% with chemotherapy alone. Subsequent to this study, combinatorial treatment with amivantamab and chemotherapy received FDA approval, for greatly improving outcomes of NSCLC when compared with conventional therapeutics.
Amivantamab has also since been approved in combination with chemotherapy for patients with NSCLC with EGFR exon 19 deletions or exon 21 L858R mutations whose disease had progressed on or after treatment with an EGFR tyrosine kinase inhibitor based on data from the MARIPOSA-2 trial.
Could Bispecific Antibodies Transform Solid Tumor Therapies?
The current prognosis for NSCLC is poor with only a 9% 5-year survival rate for metastatic NSCLC. Standard treatments, although showing some effectiveness, are associated with adverse effects, are nonspecific, and progression-free survival is suboptimal. While advances in some immunotherapies, including immune checkpoint inhibitors, have shown some promise for treating NSCLC, many patients still do not respond or recurrence occurs. However, recent advancements in the development of bsAbs, such as amivantamab, show promising signs of alternative treatments for solid tumor-based cancers such as NSCLC. Although more research is required, bsAbs are paving the way for potentially life-saving novel cancer therapies.
